Accelerate Venture Capital Fund Value Using ChronoTruth GRC Platform, ChronoSee Risk Analysis and ChronoPulse Reporting Dashboards
VC Portfolio GRC Benchmarking, Analytics and Reporting
ChronoSee and ChronoPulse is offered to the VC for their use as an ESG and GRC reporting and analytics platform
The VC would determine what portfolio companies they would like GRC analytics, including AI GRC adoption levels
ChronoSee is used by the selected Portfolio Companies to provide a status on current GRC operations. Typically taking less than an hour per quarter to perform
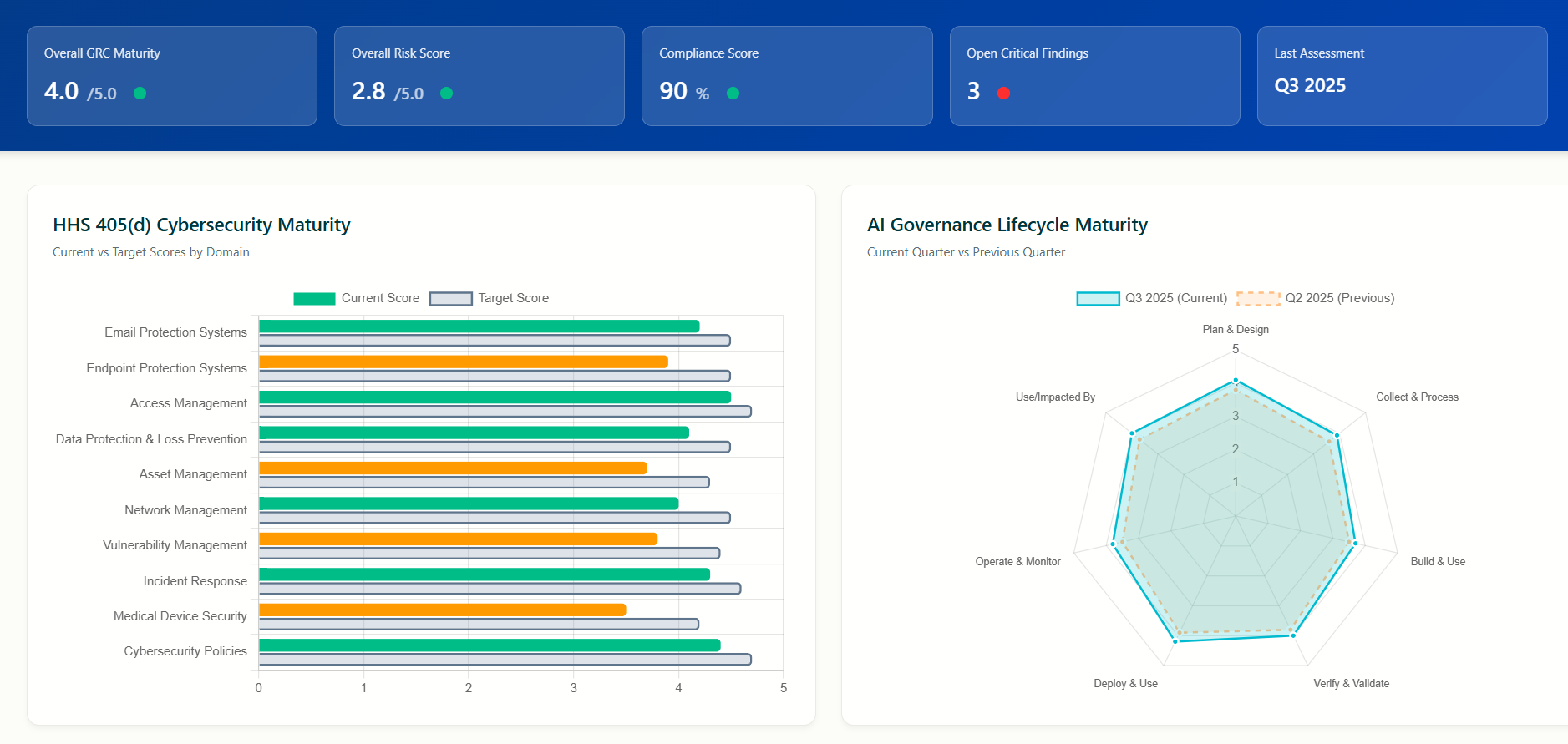
ChronoPulse provides in depth analysis on the maturity levels of each selected portfolio company using the HHS 405d assessment
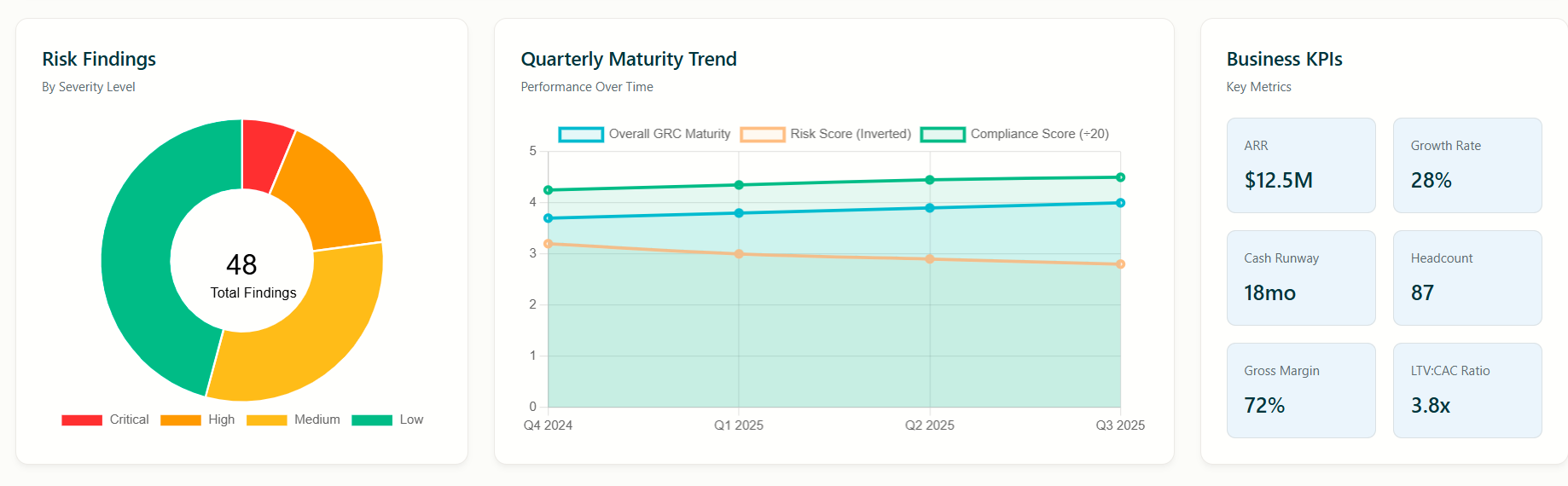
Once per quarter the portfolio company completes the ChronoSee online form based on the HHS assessment and the results are available to the VC at anytime, including historical data and trend analysis using ChronoPulse
The ChronoPulse reporting analytics uses Power BI provides detailed insights as well as Risk Simulations the VC can perform based on the reported data
Portfolio Company GRC Offering
Working with the VC, ChronoTruth is offered to portfolio companies as a Governance, Risk and Compliance platform
Accountability for Recovery and Continuity is a cornerstone of ChronoTruth in addition to Third Part Risk Management
The ChronoTruth GRC Platform is specifically designed for;
HIPAA, SOC2, FDA Section 524B, HHS 405D, ISO 14971, ISO 13485, ICH Q9 and EU MDRAI Governance, Risk and Compliance is validated using industry benchmarks aligned with each company’s’ AI operational environment.
Turn Cyber Security and Regulatory Requirements into Strategic Advantage
ChronoProof’s unique value proposition lies in transforming individual company compliance efforts into portfolio-wide governance visibility for healthcare venture capital firms

Data Sovereignty
Portfolio company data remains within its designated cloud environment, ensuring compliance with regional data residency requirements.

Compliance Alignment
Supports FDA, ISO, HIPAA, HHS, EU MDR, ICHQ9, and country-specific regulations across your entire portfolio.

ChronoPulse
Delivers tailored cybersecurity and regulatory reporting and analytics across portfolio companies.

AI Compliance Assessment
AI maturity analysis and regulatory requirements insight.

ChronoProof’s ecosystem of ChronoTruth, ChronoSee and ChronoPulse aren’t just compliance tools for individual portfolio companies—they’re strategic infrastructure investments
They empower venture capital firms to:
Have portfolio-wide visibility and aggregated reporting of standardized compliance data across diverse portfolio companies operating in different healthcare subsectors with varying regulatory obligations
Accelerate pre-investment due diligence
Portfolio company value creation
Exit readiness
Maintain centralized risk oversight across all fund portfolios
Healthcare AI compliance & ethics assessments across portfolio investments
Critical Challenges Facing Healthcare VC’s
Compliance gaps create significant downstream risk for venture investors, who may discover regulatory liabilities only during due diligence for follow-on funding or acquisition negotiations.
Regulatory Complexity and Operational Challenges Healthcare venture capital firms navigate a uniquely challenging environment characterized by regulatory complexity, high failure rates, and operational risks that differentiate the sector from traditional technology investing.
High Startup Failure Rates and Root Causes
Healthcare startups face an 80% failure rate, with 20% closing in the first year alone. This attrition stems from underestimated regulatory complexity, extended sales cycles, and the difficulty of achieving clinical validation and provider adoption.
Regulatory Compliance Gaps and Investment Risk
Regulatory compliance readiness has emerged as the top investment consideration for 70% of venture capital firms, according to industry research. Yet many healthcare startups fundamentally lack the regulatory sophistication required to navigate HIPAA, FDA pathways, state-specific requirements, and evolving AI governance frameworks.

Rapid Compliance Health Check

M&A Compliance Readiness Snapshots

Policy and Procedure Development and Integration

Custon Compliance Roadmaps

Secure Cloud Storage Environment

Third Party Risk Management Solution Set

Increase Portfolio Company Valuations

Regulatory Filing and Audit Readiness Workflow

Complete Control and Flexibility to add unique requirements

Chain of Custody for Regulatory Reporting and Due Diligence

Complete API integration for audit and regulatory evidence

ChronoTruth GUI front end with SQL database and data lake seemless interaction
ChronoSee and ChronoPulse: Cybersecurity Maturity Assessment, Analytics and Reporting
Purchased by the VC
The ChronoSee assessment comprehensively covers all 10 critical cybersecurity domains identified by the HHS 405(d) Task Group, which was formed in 2017 with over 150 cybersecurity professionals, clinicians, and public health experts from both public and private sectors.
ChronoPulse analytics and reporting provides current maturity levels in each of the 10 domains, gap analysis and corrective action plans.
Quarterly reports are presented to the VC:
Maturity level of each domain, gap analysis and corrective action recommendations
Aggregated reports by Fund and Healthcare Sector
Drill down capabilities for each company
AI implementation levels and maturity assessment
ChronoTruth: Our Purpose Built Next Generation GRC Platform
Purchased by the VC or Portfolio Company
AI Risk Modeling and Analysis, Documentation Library, Policy and Procedure Integration and Third Party Risk Management just to name a few of our solution sets within ChronoTruth, with each company having complete control to adapt to the specific environment and requirements.